Inverted Microscopy
In order to specify plankton organisms of about 10 to 500 µm size one needs to look through the microscope. This type of analys is is one of the three taxonomic methods we use along with flowcytometry and phytoplankton pigment analysis by means of HPLC (High Performance Liquid Chromatograpy). We mainly use inverted microscopes which are also good for quantitative analysis of the Phyto- or Microzooplankton in water samples. Some of the microscopes are equipped with a UV-excitation lamp, suitable objectives and filters, to perform fluorescence microscopy, which could be very helpful e.g. to distinguish between heterotrophic and autotrophic organisms. A connected camera with monitor is a good tool for teaching and outreach activities.

contact: Annegret Stuhr
Confocal microscopy – make microbial communities visible
New developments and improvements in confocal laser scanning microscopy (CLSM) in the last two decades facilitated unprecedented insight into the widely unknown microbial world. Observing bacteria, plankton, protozoan and fungi in their natural habitat is critical to understand community functions, interactions, symbioses and competitions that is essential for ecosystem function and stability. Interactions of bacteria, algae and zooplankton form the bases of the marine food web and are important in global oxygen and carbon cycles. To gain more knowledge about these microbial dynamics advanced high resolution microscopy is necessary.
The CLSM produces sharp and detailed images of any kind of surfaces. Three-dimensional structures can be visualized by imaging a series of optical sections throughout the sample and that can be set together in a high-resolution 3D image. Different components, compounds and members of marine particles, gels, aggregates and biofilms can be visualized by applying specific fluorescence dyes, probes labelled with fluorophores and markers and by using auto-fluorescence. A special application of the CLSM is to analyze gel particles, which form from dissolved organic matter and play an important role in the organic carbon cycle of the oceans, and their associated microorganisms.
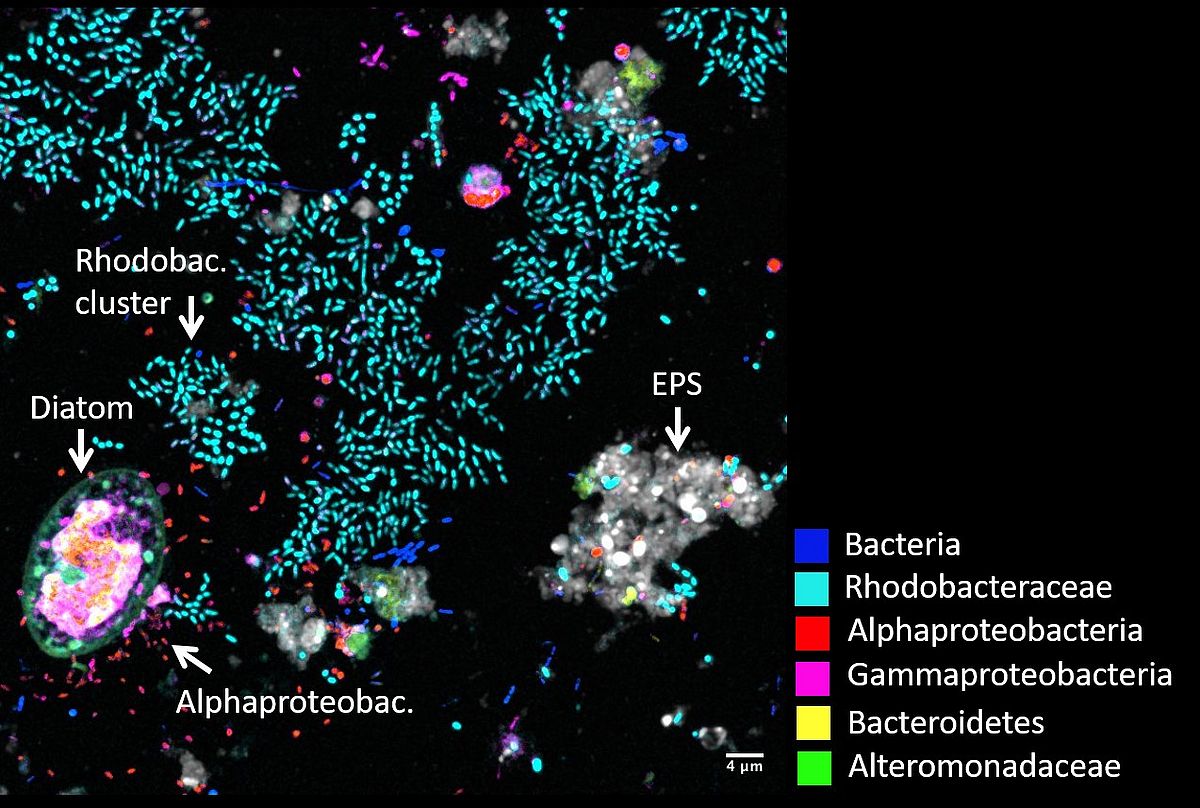
Another application of CLSM is CLASI-FISH (Combinatorial Labelling and Spectral Imaging – Fluorescence In Situ Hybridization), an advanced microscope technique that can identify the phylogeny of around 10 different microbial taxa simultaneously by keeping their spatial composition. With this technique we visualize biofilms on plastic debris in the surface ocean with 90% of the bacteria get phylogenetically identified (Schlundt et al., 2019). We observed diverse bacteria communities surrounding single phytoplankton cells probably exchanging nutrients. Within a couple of weeks, a heterogenous microbial community covers almost the entire surface of plastic debris showing that plastic is a popular substrate for biofilm formation in the ocean. We will continue to visualize and understand microbial compositions and functions on marine plastic debris and further natural and artificial substrates and particles.
Contact: Prof. Dr. Anja Engel, Dr. Cathleen Schlundt